
Author By AkshayaPosted on
The medical device industry in India has undergone significant transformation and growth over the past few years. This growth is p...

Author By Mr. Arunkumar ChokkalingamPosted on
Client Background This case study focuses on a U.S.-based medical device manufacturer specializing in endotracheal tubes. The c...

Author By AishwaryaPosted on
Purpose MD-26 is the application form used to seek permission from the Central Licensing Authority (CLA) to import or manufact...

Author By AishwaryaPosted on
Government Authority (CDSCO) The Central Drugs Standard Control Organisation (CDSCO), a Ministry of Health & Family Welfare...

Author By AishwaryaPosted on
Government Authority (CDSCO) The Indian Medical Device Regulation, established by the CDSCO, must be followed by all medical de...

Author By Ms. Sneha RamasamyPosted on
An official document issued by the Central Licensing Authorities or State Licensing Authorities that certifies a medical device is...

Author By AkshayaPosted on
The Fourth and Fifth Schedule Audits, conducted by either a Notified Body or the Central Licensing Authority (CLA), are essential...

Author By AishwaryaPosted on
In the context of medical device licensing in India, a post-submission request refers to any communication or additional documenta...

Author By Ms. Sneha RamasamyPosted on
India's burgeoning medical device market presents a lucrative opportunity for manufacturers worldwide. However, introducing innova...

Author By AkshayaPosted on
CDSCO - The Government Authority Medical device registration in India is overseen by the Central Drugs Standard Control Organisati...

Author By AishwaryaPosted on
Introduction to Technical Documentation under EU MDR 2017/745 Technical Documentation under the EU Medical Device Regulation is...

Author By Mr. Arunkumar ChokkalingamPosted on
Introduction The EU In Vitro Diagnostic Regulation (IVDR 2017/746) significantly overhauled the regulatory landscape for in vit...

Author By Ms. Sneha RamasamyPosted on
Q: What are the current audit timelines for medical device regulation, and how have they changed recently? A: The audit timelin...

Author By Mr. Arunkumar ChokkalingamPosted on
The medical device industry in India has undergone significant transformation and growth over the past few years. This growth is p...

Author By AkshayaPosted on
In the Indian medical device regulatory landscape governed by the Central Drugs Standard Control Organization (CDSCO), an "endorse...

Author By AkshayaPosted on
Incomplete or Inaccurate Information: Missing Documents: Not including all the required documents as per the CDSCO guidelines...

Author By Mr. Arunkumar ChokkalingamPosted on
What is EUDAMED It's a European Data bank on Medical Devices, is a key element under the EU Medical Device Regulation (MDR 2017...

Author By AkshayaPosted on
Class A non-sterile non-measuring medical devices - Final notification GSR 777(E) was released by the Indian Ministry of Health an...

Author By Ms. Sneha RamasamyPosted on
Understanding ISO 13485 and Notified Bodies ISO 13485:2016: The internationally recognized standard for quality management sys...

Author By AkshayaPosted on
By 2025, the rapidly expanding Indian healthcare sector will generate $280 billion in revenue. One of the top 20 medical device ma...

Author By Mr. Arunkumar ChokkalingamPosted on
In India, the form for obtaining permission to import or manufacture a new In-Vitro Diagnostic (IVD) medical device is MD-28. Here...
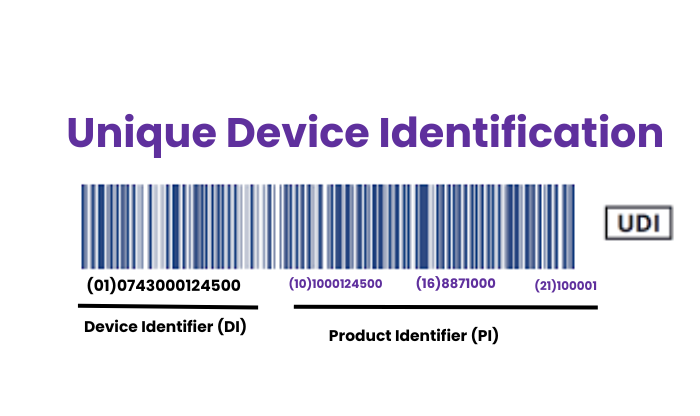
Author By Mr. Arunkumar ChokkalingamPosted on
Definition : The UDI is a series of numeric or alphanumeric characters that provides a globally unique identifier for medical dev...

Author By Mr. Arunkumar ChokkalingamPosted on
Technical Documentation under the EU Medical Device Regulation is a thorough collection of documents proving a medical device's co...
Author By Mr. Arunkumar ChokkalingamPosted on
It's a European Data bank on Medical Devices, is a key element under the EU Medical Device Regulation (MDR 2017/745) and the In Vi...
